
In recent years, radiopharmaceuticals have shown strong clinical potential, and the clinical demand for nuclear drugs has shown an obvious upward trend. According to the data of the Chinese Nuclear Medicine Annual Meeting in 2023, it is expected that China will usher in the demand for nuclear medicine every year of nearly 100 million people in the future. The supply demand of nuclear drugs is increasing rapidly, and the safety, reliability, efficiency and stability of therapeutic nuclear drugs are also facing higher challenges. At present, the manual preparation commonly used in the production of nuclear drugs no longer meets the needs of nuclear drug preparation, especially the demand for high-dose production of nuclear drugs at the enterprise level, and the industry needs to introduce new ideas for industrialization to change the status quo.
In September 2023, Wuxi Sunmao Medical Technology Co., LTD. (hereinafter referred to as Sunmao), the original high-end intelligent nuclear drug equipment brand of Norroy, announced that its first self-developed nuclear drug automation production line completed installation, debugging and related validation in the GMP nuclear drug production workshop. And successfully completed the manufacturing process of 177Lu-NY108 (prostate cancer radiation therapy drug) IND application.

At present, in the successful completion of the 177Lu-NY108 drug production process, the production line can complete a single batch of 1Ci or more drug production, automatic synthesis process with high yield, good quality and stability, meet the requirements of audit tracking, can be checked and printed production reports at any time, providing a strong guarantee for IND applications.
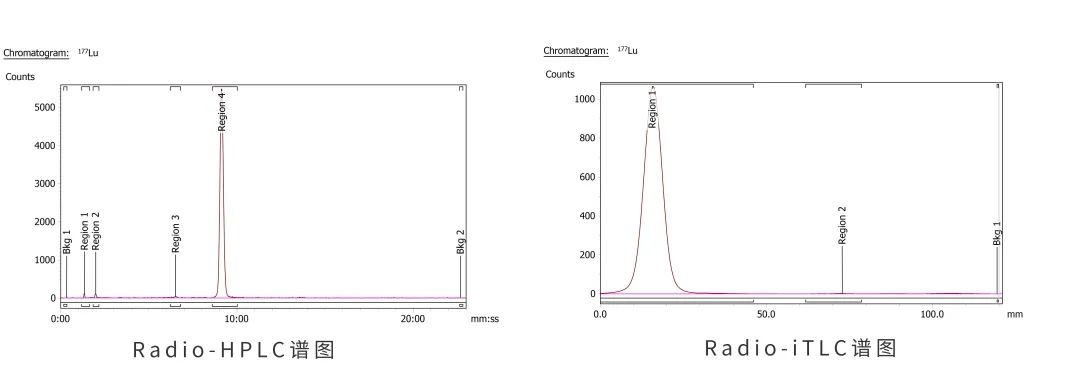
In the nuclear drug IND application stage to achieve the automation of the production process, there is almost no experience to follow in China. In the research stage, the innovative nuclear drug enterprises often adopt the way of manual preparation for synthesis. This mode is highly dependent on the operator's technology and experience, and the operation steps are cumbersome, the risk of radiation exposure is high, and the stability cannot be guaranteed. The GMP grade nuclear drug production line designed by Sunmao has provided more choices for radiopharmaceutical companies.
This automated production line is designed and built by the senior R&D team of Sunmao with the advantages of highly mastering the industrial process of automated nuclear drug production. Through process upgrading to optimize the equipment structure of the production line, and equipment precision control to improve the process level, while solving the problem of trace metal impurity pollution in the production line pipeline, to achieve GMP level production. With the standardized operation platform, it fully meets the requirements of the nuclear drug production line, and provides multiple guarantees for the improvement of the stability of nuclear drug production, the shortening of the delivery cycle, and the improvement of production efficiency.
Standing at the turning point of the development of nuclear medicine innovation, Sunmao first explored practice, and injected new vitality into the goal of promoting the transformation of nuclear medicine production to automation. In the future, Sunmao will continue to promote the construction of other automated production lines, continue to innovate in order to better meet the needs of industrial production, empower the development of customers, and contribute more to the nuclear medicine industrialization 4.0.
 86-0510-88786189
86-0510-88786189Address: No.35-208, Changjiangnan Road, XinWu District, Wuxi, China
Mail address: bd@norroybioscience.com
Website: www.adm5.cn

